Our Technologies
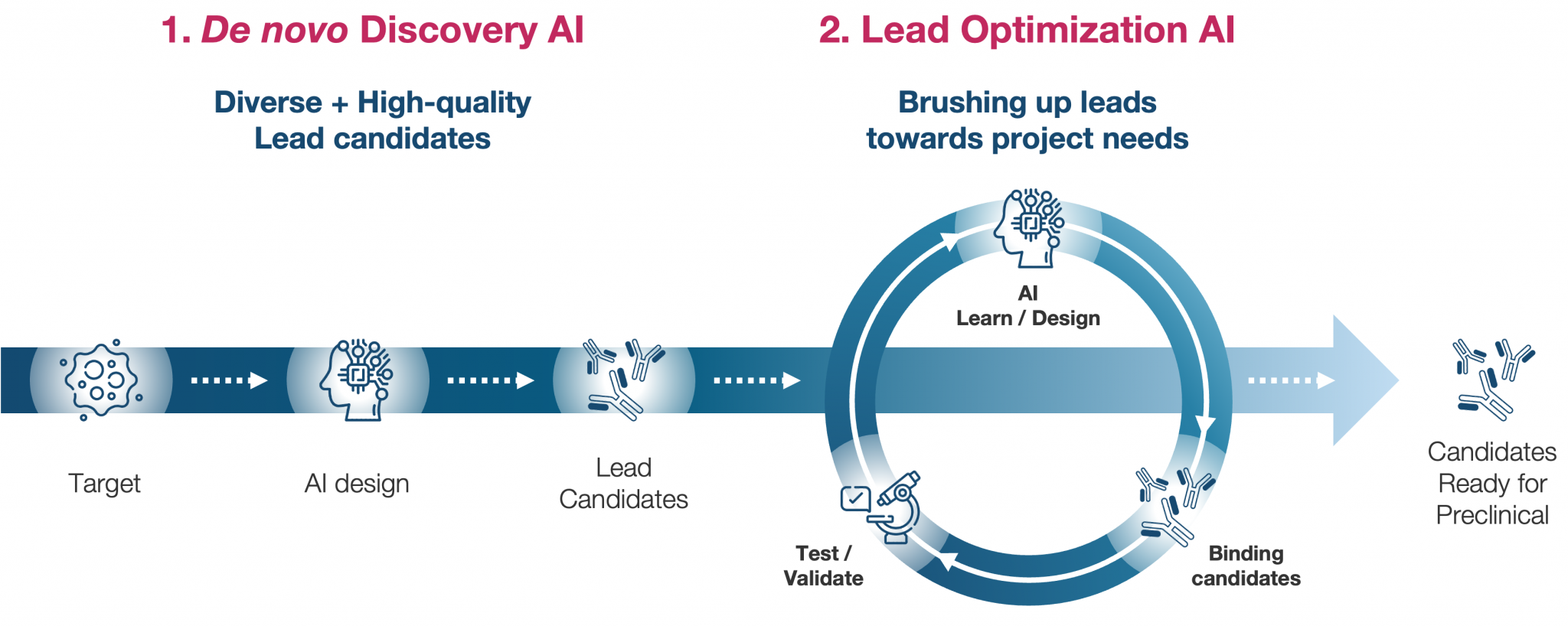
AI Antibody Discovery Platform
MOLCURE’s Core Technology and Strengths
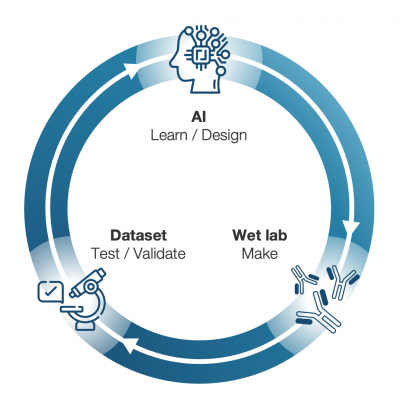
Core Technology
- An evolving AI platform with an in-house rapid learning loop
- Specialized algorithms for building AI suitable for biological data
Strengths
- Proprietary Antibody LLM: Large Language Model build from scratch
- Proprietary Database: Over 1 billion proprietary datapoints, Antibody / Peptide
- Wet lab: Directed Evolution Experiment, Next Generation Sequencer
Company Presentation by our CEO/CSO, Dr. Tamaki (at PEGS Boston 2025)
For details, please see here.
How We Collaborate
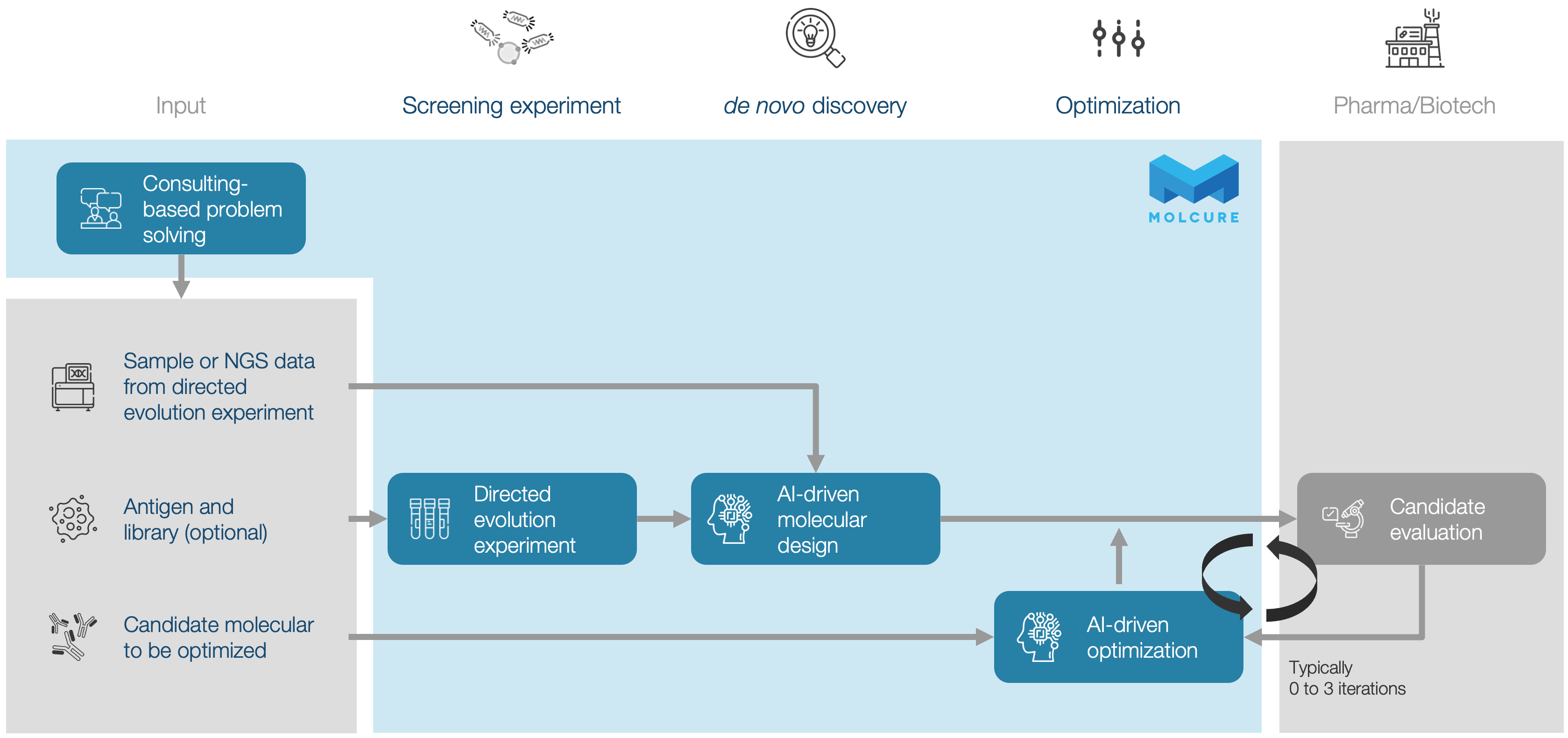
Screening, NGS, and AI Analysis
For partners seeking end-to-end support in AI-driven molecular design, including high-throughput screening, next-generation sequencing (NGS), and advanced AI analysis.
AI-Powered de novo Discovery
For partners aiming to discover novel molecules from their samples using MOLCURE’s proprietary AI technologies.
AI-Driven Lead Optimization
For partners who wish to optimize their own candidate molecules through MOLCURE’s AI-based lead optimization platform.
Consulting-Based Problem Solving
For partners looking for expert guidance on leveraging AI technologies to accelerate and enhance their drug discovery processes.